Description
TruScreen’s breakthrough cervical cancer screening device is a viable and attractive alternative to traditional cervical screening methods. The TruScreen device resolves many of the ongoing issues associated with traditional methods, such as PAP tests and cytology, including failed samples, poor patient follow-up, patient discomfort, and the need for supporting laboratory infrastructure.
It also resolves the problems associated with HPV testing, which tests for the much more prevalent HPV virus, and not the presence of pre-invasive cervical cancer states. These issues are particularly pertinent in low- and middle-income countries (LMICs) – key markets for TruScreen.
World class technology, made simple.
TruScreen uses unique, proprietary Opto-Electrical technology to evaluate the tissue of the cervix. Unlike cytology, TruScreen does not only examine surface epithelial cells.
Specific frequencies of light transmitted through the cervical tissue to identify changes in the basal and stromal layers. 4 LEDs sequentially emit light at 3 wavelengths, distant red, infrared and green.
Electrical measurements test the cell’s resistance to current to characterise the tissue. This characterisation is called electrical impedance spectroscopy.
Despite its sophisticated technology, the TruScreen device is easy to learn and operate*.
The TruScreen cervical cancer screening device is smarter than it looks.
Over 23 man-years were spent on algorithm development and testing to arrive at the current version.
The optical and electrical measurements taken throughout a screening are analysed by an integrated AI-enabled algorithm.
The AI-enabled algorithm will determine if the cervical tissue is “normal” or “abnormal” (Pre-cancerous/cancerous). The device completes this analysis immediately providing the patient’s objective results at the point of screening.
How does the TruScreen device work?
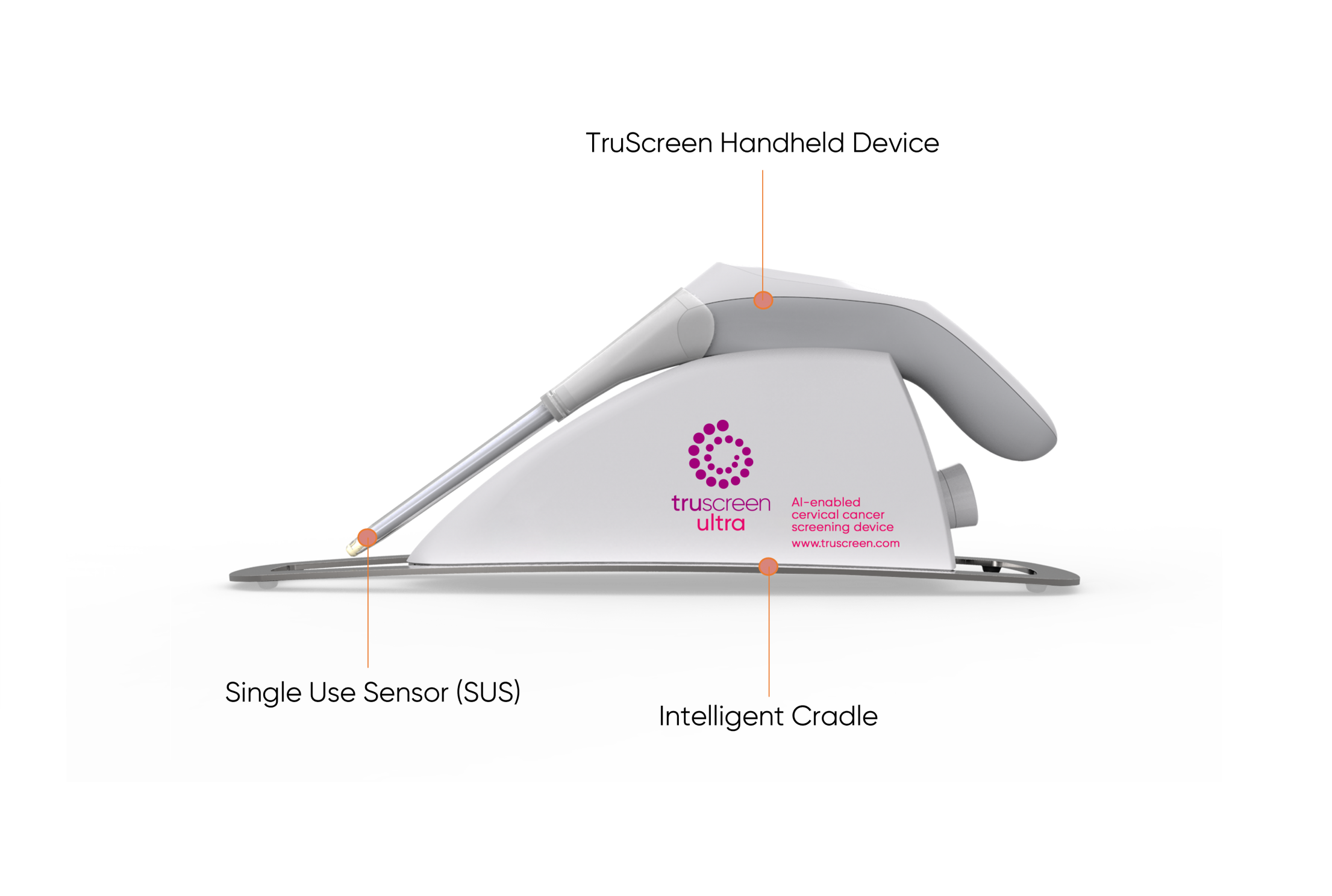
The TruScreen device consists of a disposable Single Use Sensor (SUS), a Handheld Device (HHD), and an Intelligent Cradle (IC) which works together to detect and identify cancerous and precancerous changes to the cervix.
Firstly, a pen-like wand covered by a Single Use Sensor (SUS) is used to gently touch multiple spots on the cervix. The SUS contains a precision lens and electrodes which interfaces with the cervix. In doing so, it sends and picks up low level electrical and optical signals (14 readings per second) from the cervical tissue.
The TruScreen Handheld Device then acts as an integrated AI-enabled algorithm to analyse these signals and compares them to an integrated database of 2,000 patients drawn from a wide range of geographic and ethnic backgrounds with differing histological diagnoses. This analysis identifies the presence of abnormal (cancerous and pre-cancerous) cells in the cervix and provides physicians with real-time results.
Each TruScreen examination takes one to two minutes to produce results, compared to conventional Pap tests which can take weeks, or even months in some countries, for a result to be returned.
How does screening with TruScreen compare to other methods?
The TruScreen device has been tested extensively in numerous studies around the world. Clinical studies have shown its performance is equal to, or better than, high-quality cytology tests.
The TruScreen device’s instant reporting eliminates delays associated with transporting samples to laboratories for analysis, thus avoiding the risk of losing patient contact.
Unlike cytology-based screening which requires laboratory infrastructure and involves high resource costs, the TruScreen device can be used effectively by medical or paramedical staff, with minimal training.*
An ongoing study with the Chinese Obstetrics and Gynaecology Association (COGA) comparing TruScreen to Liquid-Based Cytology (LBC) and HPV DNA testing (HPV) showed TruScreen has exceeded expectations.
Interim results demonstrated TruScreen has a sensitivity of 89.29% (compared to 67.87% for Liquid-Based Cytology (LBC) and 92.86% for HPV tests) and a specificity of 87.17% (LBC 90.07%, HPV 87.17%).
Sensitivity and specificity are measures of a test’s ability to correctly diagnose a disease. A high sensitivity results in fewer false negatives, and a high specificity results in fewer false positives in the screening process.
The TruScreen device is not only an accurate system for cervical cancer screening, but importantly for LIMICs, it is a portable device that can be used with minimal training and without the infrastructure associated with traditional cytology screening tests.*
Additionally, unlike Pap tests, the device does not collect tissue samples meaning patients experience minimal discomfort and pain. This means patients are more likely to return for repeat screenings thereby improving overall screening rates.